During the 1990s molecular biologists were fully engaged in a race to determine the complete DNA sequence in various organisms. And they succeeded�first in bacteria, then in yeast, and finally in a well-researched roundworm (C. elegans). In early 2000 the DNA sequence of the fruit fly, the genetic workhorse of the twentieth century, was completed. In June, 2000, at the White House amid media fanfare, two genome sequencing teams announced that they had completed a "working draft" of the human genome. Their reports were published in February, 2001 (1,2). The mega-project was at an end�or was it actually just the beginning?
But Gilbert saw more in the sequence completion than virtually endless strings of letters on a computer screen, representing nitrogenous bases in DNA. He spoke of gaining "total knowledge of the human organism." This statement reflects a tendency�one that seemed to accelerate in stride with gene-finding�to make overblown claims about the genome work. We might expect such hyperbole from the media seeking the hottest stories, but the scientists involved in the work were often the worst transgressors of measured assessment. The genome project was, in the words of the public-team leader, Francis Collins, "the most important and most significant project that humankind has ever mounted" (4). Why? Because it meant opening what he, like many others, called "the book of life," a book that reveals the secrets of the human being. "For the first time," stated biologist Robert Weintute, "we are reducing ourselves down to DNA sequences...to rather banal biochemical explanations....We are dealing with the mystery of the human spirit" (5).
When the New York Times announced in its June 27, 2000 headline that "Genetic Code of Human Life is Cracked by Scientists," the lead article proclaimed: "In an achievement that represents a pinnacle of human self-knowledge, two rival groups of scientists said today that they had deciphered the hereditary script, the set of instructions that defines the human organism." Interestingly, at this pinnacle of fervor concerning the project, some scientists were markedly more circumspect in their comments. Molecular biologist David Baltimore remarked, "we've got another century of work to figure out how all these things relate to each other" (6). Another scientist spoke of the genome as an "internal scaffold for our existence" (7). And still another stated, "It's like a book in a foreign language that you don't understand. That's the first job, working out the language" (quoted in 8).
These scientists are telling us that the genome project was actually just the beginning of real understanding. It is, after all, one thing to find a scaffold or a book that you haven't even begun to decipher (and we should remember, in applying the book metaphor, that a book is not the thing itself, but only a reference to the actual content). It is a wholly different matter to gain knowledge of the actual workings of the living organism, not to mention self-knowledge and finding a key to "the mystery of the human spirit."
So was the genome project just caught up in one big jamboree of hype? In many ways, yes. In a letter to the editor of Nature, written before the completion of sequencing was announced, scientist Sol Hadden puts his finger on some essential issues:
Current hype about the expected completion of the Human Genome Project demands some clarification. Although initially conceptualized more broadly, the project is effectively about determining the sequence of bases in the human genome. This is not the same as trying to understand the program that is encoded in human DNA. Consequently, the results will be in the merely descriptive naturalistic tradition. Technical development has always had that effect on scientific disciplines, for example the electron microscope, the radio telescope or the automated DNA sequencer.In other words, the human genome project really serves to show how little we know. And we could have realized all along�if hype did not have such a strong pull on us�that reams of data (2000 New York City telephone books' worth) would not tell us much. The real challenge is to understand genes in the context of the living organism and not to connect this endeavor with the expectation that such knowledge will open up the secrets of life.Of course, researchers are always quick to emphasize the importance of their work to whatever application is in vogue, and curing disease is a worthy goal. But how will the Human Genome Project help to achieve this end? A look at any [gene map] from any species reveals what looks like an explosion in a slaughterhouse. Where is the order we need, to make sensible rather than trial-and-error genetic manipulations?
In any case, pharmacogenomics [using genetics to make medicines] requires an understanding of the apparent genetic 'disorder' in any organism's genome, of genotype-phenotype mapping, of gene-gene interactions, of intraspecific genetic variability, and of self-organizational processes, rather than endless lists of DNA bases. (9)
The real question is, however, why did anyone think that genes make an organism what it is in the first place? As biologist Svante P��bo comments, successes in the last decade
have resulted in a sharp shift toward an almost completely genetic view of ourselves. I find it striking that 10 years ago, a geneticist had to defend the idea that not only the environment but also genes shape human development. Today, one feels compelled to stress that there is a large environmental component to common diseases, behavior, and personality traits! There is an insidious tendency to look to our genes for most aspects of our "humanness," and to forget that the genome is but an internal scaffold for our existence. (7)What is so strange about the genocentric view is the fact that the genetic discoveries themselves don't actually support it. The results are simply being viewed through a deterministic and materialistic lens.
In 1994, Walter Gehring's research group in Basle, Switzerland, discovered that the human being, mouse, and fruit fly all have a gene�called Pax 6�that is not only very similar (homologous) in each species, but is also related to eye formation (11). This came as a surprise, since the eyes of mammals and insects are totally different anatomically. No one expected the "same" gene to be related to such different structures.
The apparent connection between the Pax 6 gene and eye development became more compelling when researchers were able to manipulate fruit flies to express the Pax 6 gene in tissues that would normally become wings, legs, and antennae (12). The result was wholly abnormal fruit flies with partial eyes growing on their legs and wings and even on their antennae. In compensation, these parts often did not develop fully. The scientists then proceeded to do the same experiment with the homologous Pax 6 gene from the mouse. The fruit flies again made eyes�fruit fly-type and not mouse-type�on other body parts. The same experiment succeeded with Pax 6 genes from sea squirts and squids. Gehring concluded that they had clearly discovered and demonstrated the existence of a "master control gene" for eye development (12,13).
But, as is usually the case in biology, the story and the conclusion are not so straightforward. Since the Pax 6 gene is in yet unknown ways functional in animals without eyes, like roundworms and sea squirts, it is clearly not related to eye development in these organisms. In other organisms it is also connected to different developmental processes. Mutant mice with two copies of the altered Pax 6 gene not only have no eyes at all, but they have malformed noses, cannot breathe, and die. In squids the gene is active in tentacle formation. In the fruit fly it is involved in the development of other parts of the nervous system beside the eye, and if the Pax 6 gene is not expressed at all in mutants, they die. And in the fish-like lancelets (amphioxus), it is related to the development of olfactory and central nervous system tissue.
So, it seems that, in each organism where it has been found, the "master control gene" for eye development is involved in processes other than eye development. Within a particular organism it is active at different places and at different times, depending on the organ or tissue that is forming there and then (see Figure 1). Evidently, it's not just the gene that determines the function.

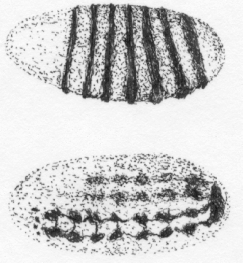
Figure 1. One gene, different functions. The FTZ gene in the fruit fly is needed to form a particular protein (the fushi tarazu protein). But the gene and this protein have more than one function during the fly's embryonic development. The drawings show two fruit fly embryos, one at an earlier (top), the other at a later stage of development (bottom). The dark stripes and blotches represent the FTZ protein, which was made visible by staining. In the earlier stage (top) this protein is expressed in bands and active in the formation of segment boundaries; it is then broken down. Only three hours later (bottom), the protein is formed anew and is involved in the development of nerve cells. Thus the FTZ gene is first a "gene for" segment development and then a "gene for" nerve cell development. (Redrawn from 14)
This fact led Denis Duboule and Adam Wilkins to use the term, "bricolage," to express how the organism uses what is genetically at hand to realize its own specific development. They expect that "the primary source of developmental differences between fruit flies and foxes will prove to be not unique genes but rather the way that comparable, or the same, gene functions are differently deployed in their development" (14).
A recent experiment illustrates this fact clearly (15). The lancelet (amphioxus) is a close relative to the vertebrates and is often used to depict how the evolutionary ancestor of vertebrates might have appeared. It is a small fish-like creature that has, however, no bony skeleton and no paired fins (see Figure 2). Its front end is pointed, and biologists don't speak of a head because typical head features, like brain and brain capsule, developed sense organs, or a jaw, are missing.
Scientists have found a group of developmental genes, called the Hox genes, that are related, among other things, to the formation of head structures in vertebrates. These Hox genes were also discovered in the lancelet, and since it is has no head, these genes must be related to other, up till now unknown, processes in lancelet development. When, however, the sequences that regulate lancelet Hox gene expression were implanted into mice and chick embryos, they turned out to control genes in head-forming tissues. This means that a DNA sequence with specific functions in one organism can be utilized by another organism to form completely different tissues and organs.
Both this and the "eye" gene example show us that genes don't make the organism. What a gene "is," is dependent on the organism in its spatially and temporally unfolding existence. You always have to presuppose the organism to understand the gene. This conclusion has far-reaching implications.
Take, for example, our conception of evolutionary processes. The scenario taught in schools and universities around the world is: The gradual accumulation of gene mutations causes organisms to evolve new characteristics. But this scenario doesn't work, if we take the results of developmental genetics seriously. Rather, we must imagine the evolving organism utilizing "old" genes in new ways to realize new evolutionary developmental characteristics. This view removes genes from their pedestal in evolutionary theory, since they can no longer be seen as the driving evolutionary force. The whole organism�which has been virtually lost in genetic and evolutionary thinking today�returns to the center stage of development and evolution.

Figure 2. The lancelet (amphioxus) is a fish-like animal that dwells in coastal waters and burrows into sand. About two inches long, it feeds by straining small organisms out of the water.
The work on viruses and bacterial cells that gave birth to molecular biology in the 1940s and 1950s significantly strengthened the earlier notion of "one gene, one function" of early mendelians. Furthermore, in recent decades, geneticists and molecular biologists have inadvertently contributed to this misconception by the ways they name their genes based on how they were first identified�breast cancer genes, growth factor genes, and so on. This semantic imprecision has had an unfortunate effect on public perception of gene action: many lay people apparently believe that phenotypic traits, such as blue eyes or obesity, are due to the exclusive function of particular genes.... Explicit recognition of the general rule of multiple use of specific regulatory gene products would help to clarify issues in both development and evolution. (14, p. 56)Just as the 1990s were the decade of genome sequencing, so also were they the decade in which hardly a day went by without an announcement of the discovery of a new gene determining some trait: longevity, happiness, day-night rhythm, alcoholism, schizophrenia, sex drive, Alzheimer's and even IQ. It's no wonder everyone believes that we're determined by our genes.
But if the working of genes is complex and subtle, as the research we've described shows, then something must be awry in the claims about finding genes "for" this or that trait. Geneticists Neil Risch and David Botstein wrote a commentary in Nature Genetics in 1996 describing the search for the gene for manic depression (16). They found that over the previous twelve years sixteen different research groups had announced the discovery of genetic linkages to manic depression (which translates in popular language into "gene for manic depression"). The problem�from a "one-gene, one trait" perspective�was that these researchers identified fifteen different locations for the gene on eleven different chromosomes! Not lacking in humor, Risch and Botstein state that "one might argue that the recent history of genetic linkage studies for this disease is rivaled only by the course of the illness itself." They see the lack of consistency as an expression of the complexity of the illness on the one hand and not enough rigor in statistical analysis on the other. Evidently, the urge to find a genetic cause often overshadows the recognition of the complex nature of the phenomena.
As we have described elsewhere, even diseases that follow a more straightforward Mendelian pattern of inheritance, like sickle-cell anemia, are complex when looked at more carefully (17). It doesn't take much investigation to find that all of the characteristics or diseases listed above�none of which follow a Mendelian pattern�are strongly related to individual and environmental factors, as well as having some hereditary component.
The problem is that the isolation of a genetic factor is always based on a narrow theoretical and experimental framework. Or to put it in Kurt Goldstein's terms, genetics works with the method of isolation and therefore produces results that are valid only within that framework (18). Take the example of amphetamine susceptibility. Scientists discovered that two different inbred strains of mice showed a very different relation to amphetamines: strain C mice preferred the box where it received injections of amphetamine, while strain D mice avoided this box (19). You can already picture the headlines: "Scientists prove amphetamine addiction is hereditary." (How often we read such articles only to discover that what we thought was a report about a human condition turns out to be an experiment with rats or fruit flies!)
But in this case the scientists were very careful and performed an additional experiment: they gave the mice less food over a period of time, while continuing amphetamine injections. Something unexpected occurred: Strain D mice began to prefer the injection box, while the previously "addict-type" strain C mice avoided the box. A total reversal of the results! This example illustrates drastically what, in fact, is generally the case: a "fixed genetic predisposition" may actually be only one of many appearances (phenotypes) of an organism, and this particular appearance depends largely on the specific experimental and environmental circumstances under which the trait was observed.
And this will occur in the name of human rationality. In 1998 a group of scientists met to discuss genetic manipulation of human beings, and the proceedings were published two years later (20). The participants promoted the view that science must progress and that genetic modification of human beings is inevitable. "Science proceeds and succeeds by doing....what we're talking about here are incremental advances with enormous implications" (20, p. 80). James Watson, the co-discoverer of the double-helix model of DNA and the first head of the Human Genome Project, made the following comment:
Some people are going to have to have some guts and try germline therapy without completely knowing that it's going to work.... And the other thing, because no one has the guts to say it, if we could make better human beings by knowing how to add genes, why shouldn't we do it? What's wrong with it? Who is telling us not to do it? I mean, it just seems obvious now.... If you could cure what I feel is a very serious disease�that is, stupidity�it would be a great thing for people who are otherwise going to be born seriously disadvantaged. We should be honest and say that we shouldn't just accept things that are incurable. I just think, "What would make someone else's life better?" And if we can help without too much risk, we've got to go ahead. (20, p. 79)Watson is known for his blunt statements, revealing, we believe, a widespread sentiment that other scientists share, but don't dare to express: the path of genetic engineering leads to the human being, and we shouldn't close our eyes to this inevitable fact. The real challenge, in this view, is to convince the public. The book's editors, scientists Gregory Stock and John Campbell, write:
To think rationally about ethical issues in germline engineering requires basic understanding of inquiry-based analysis and general scientific (biological) background.... If all scientists were to make a commitment to improving K-12 science education in their local communities, we might eventually have a society capable of thinking analytically and rationally about the challenges and opportunities of science�including germline engineering. (20, p. 24)In other words, people are not smart enough to see where science needs to take humanity. If we could get all elementary school children to isolate genes, middle school children to sequence them, and finally high school students to manipulate organisms with the genes, then we'd have the proper preparation. Of course, all learning about living organisms in their natural habitats would have to be dropped to provide space for such a high-tech curriculum. This would be the way to further "rational thinking."
In reality, what Stock and Campbell are aiming at is indoctrination in reductionism, so that people will lose the capacity to see through the weak and outlandish arguments of a Nobel laureate like James Watson. It's astounding that we've come so far that being rational is equated with tearing a narrow, genetic segment from the fabric of life and treating it as though it were everything. You're rational if you restrict yourself from seeing how your sector of knowledge relates to a larger whole.
As we have shown, the results of modern genetics are shouting at us to wake up and see that we've got to start taking the whole organism seriously and view genes in light of the organism and not only the other way around. Genetics began by defining genes in relation to a particular trait, ignoring the experimental and conceptual framework, and also ignoring the organism as a dynamic, changing entity. Now the emphasis should be on how an organism utilizes its genes within this broader context. Goethe would be happy, knowing that even the paramount reductionist science is showing�if not consciously recognizing�that he was right in emphasizing the "how" of nature and not just the "what."
But the reductionist path is well worn and deeply entrenched. Once you're in it, it's hard to climb out. It's not easy to break out of habits and change an inner direction. It means giving up the security that comes with focusing on our own particular program that biases the mind from the outset. ("Understanding an organism means reducing its functions to underlying mechanisms.") Instead, our focus needs to be on entering the richness of the phenomena themselves and changing our viewpoints in order to do justice to what we discover. Instead of barraging the world with a monologue, we enter into conversation with it. How else can we hope to find deeper understanding and responsible ways of acting?
Craig Holdrege is director of The Nature Institute. Johannes Wirz, a molecular biologist, is on the staff of the Research Laboratory at the Goetheanum in Dornach, Switzerland. Craig and Johannes have worked together many years developing a contextual approach to genetics.
2. Venter, J.C. et al. (2001). The Sequence of the Human Genome. Science 291:1304-1351.
3. Gilbert, W. (1991). Towards a Paradigm Shift in Biology. Nature 349:99.
4. Quoted in New York Times, November 30, 1993, p. C1.
5. Quoted in New York Times, March 10, 1998, p. F1.
6. Quoted in New York Times, June 27, 2000, p. A1.
7. P��bo, S. (2001). The Human Genome and Our View of Ourselves. Science 291:1219-1220.
8. Pennisi, E. (2001). The Human Genome. Science 291:1177-1180.
9. Hadden, S. (2000). How Much Use is the Human Genome Project? Nature 406:541-542.
10. Claverie, J-M. (2001) What if There Are Only 30,000 Human Genes? Science 291:1255-1257.
11. Quiring, R. et al. (1994). Homology of the eyeless Gene of Drosophila to the Small eye Gene in Mice and Aniridia in Humans. Science 265:785-789.
12. Halder, G. et al. (1995) Induction Of Ectopic Eyes by Targeted Expression of the eyeless Gene in Drosophila. Science 267:1788-1792.
13. Gehring, W. and Ikeo, K. (1999). Pax 6: Mastering Eye Morphogenesis and Eye Evolution. Trends in Genetics 15:371-377.
14. Duboule, D. and Wilkins, A. (1998). The Evolution of "Bricolage." Trends in Genetics 14:54-59.
15. Manzanares, M. et al. (2000). Conservation and Elaboration of Hox Gene Regulation During Evolution of the Vertebrate Head. Nature 408:854-856.
16. Risch, N. and Botstein, D. (1996). A Manic Depressive History. Nature Genetics 12:351-353.
17. Holdrege, C. (1996). Genetics and the Manipulation of Life: The Forgotten Factor of Context (see especially chaps. 3, 5, and 6). Hudson, N.Y.: Lindisfarne.
18. Goldstein, K. (1963, originally published in 1939). The Organism. Boston: Beacon Press. (A reprint was published in 1995 by Zone Books in New York.)
19. Cabib, S. et al. (2000). Abolition and Reversal of Strain Differences in Behavioral Responses to Drugs of Abuse After a Brief Experience. Science 289:463-465.
20. Stock, G. and Campbell, J. (2000). Engineering the Human Germline. New York: Oxford University Press.
This document: http://www.netfuture.org/ni/ic/ic5/genome.html
Original
source: In Context (Spring, 2001), published by The Nature Institute
Go to The Nature
Institute's main page
Go to The Nature
Institute's publications page